Research topics
We believe that smarter drug delivery systems can extract maximum benefit from active pharmaceutical
ingredients (APIs) resulting in better therapeutic outcomes and improved quality of life for patients. By
combining the knowledge of pharmaceutical sciences and material engineering, we aim to deliver APIs
to the site of action when needed and in concentration needed. The efficacy of the therapy can thus be
enhanced, while side effects from exceeding therapeutic concentrations or therapeutic failure due to
insufficient drug concentrations can be minimized. Better drug delivery systems make a healthier future.
Development of novel drug delivery systems (electrospun, printed and liposomal DDSs)
Comprehensive understanding of the material and process characteristics is crucial for the implementation of novel technologies. Thus, our research aims to improve the understanding of these factors starting from the molecular level and solid-state properties of the materials, influence of process parameters, and coming to see how these define the structural properties of produced materials and how it all affects biopharmaceutical properties that finally translate into biological responses.

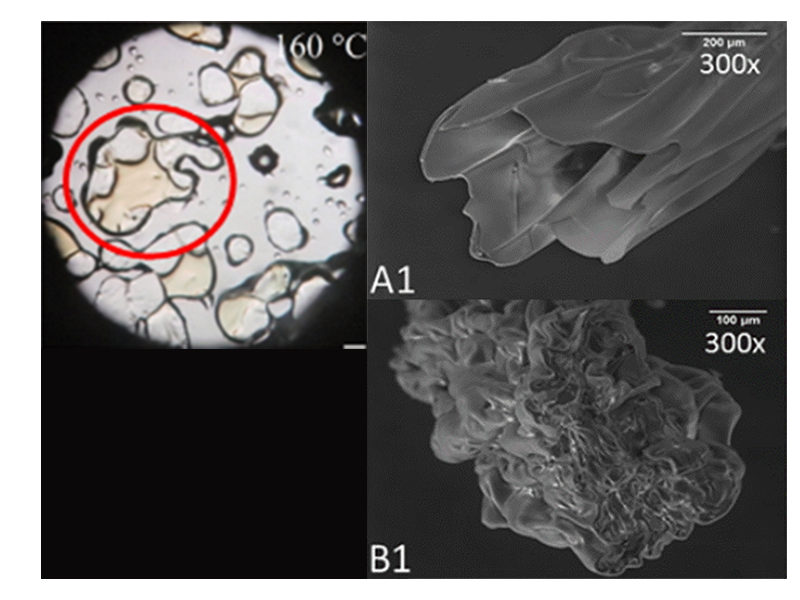
Design and development of nano- and microfibrous antimicrobial drug delivery systems for wound healing applications

Design and development of nano- and microfibrous antimicrobial drug delivery systems for oral infections (periodontitis)
Oral cavity represents a challenging setting for wound healing due to bacteria-laden environment and constant physical trauma. Localized antimicrobial treatment of oral wounds and infections, e.g., periodontitis, offer several advantages over systemic administration, such as allowing higher antibiotic levels while avoiding systemic toxicity, prolonged retention of antibiotic at the target site with the consequent decreased possibility of emergence of bacterial resistance, and enhanced patient compliance. Recognizing these advantages, we develop electrospun antimicrobial DDSs for the local treatment of oral wounds and infections.

Development of biorelevant analytical techniques and wound infection models
For example, conventional drug release testing methods do not reflect the wound environment adequately and cause difficulties for correlating and predicting biological activity from the dissolution data. We have developed biorelevant hydrogel-based drug release and diffusion assays using extraction/HPLC, UV-imaging and bacterial bioreporters for detection. The latter provides an additional benefit of simultaneously monitoring antibacterial activity of the released drug. Also, we put special emphasis on designing wound infection models with sessile bacteria in biofilm as it is recognized as a major culprit in therapeutic failure of non-healing wounds.

Solid state analysis and phase transformations
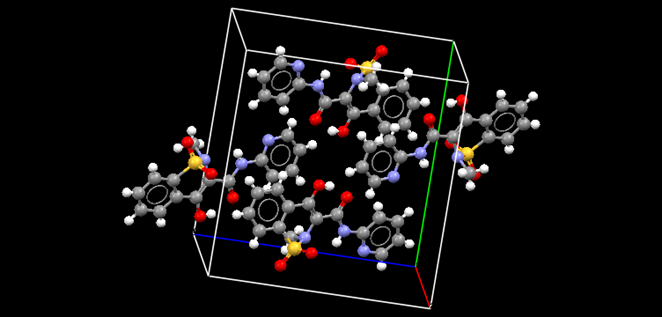
Design and preparation of amorphous solid dispersions
Poor aqueous solubility is a major problem for drug development, therefore novel pharmaceutical technology approaches are sought to improve this. Preparation of amorphous solid dispersions (ASDs) is one of the possible solutions for this problem as amorphous drugs have improved apparent aqueous solubility and higher dissolution rate. The main scientific scope of the research work is to gain understanding of the solid-state transformation phenomena when preparing ASDs of poorly water-soluble drugs, and to exploit this knowledge in the development of stable formulations consisting of amorphous drug. Within ASDs the drug is stabilized in its amorphous form within a polymer matrix. Different processing and formulation-based methods, such as electrospinning (ES), milling at variable temperatures, quench cooling of co-melts and solvent evaporation, are used to prepare ASDs of poorly water-soluble APIs. Improved stability and dissolution behavior of ASDs together with better in vivo biopharmaceutical performance in rats has been demonstrated. Deeper investigation is performed to understand the miscibility of the ASDs and two-phase ASD systems. API-polymer interactions at the molecular level are investigated which significantly influence the stability of ASDs. Excipients are a relevant part of the pharmaceutical formulation and all excipients used in a dosage form preparation need to have a specified functionality. Novel substances are tested to be used as potential new pharmaceutical excipients (e.g. lignin, lignocellulose). Drug-excipient interactions during in vitro dissolution testing as well as in vivo animal studies have been investigated.